Download PDF
Michaela Rektorysova
School of Medicine
Faculty of Medical and Health Sciences
University of Auckland
Abstract
Non-menopausal women have a lower incidence of hypertension and
cardiovascular complications compared to age-matched men. This
cardiovascular advantage is thought to be the result of oestrogen’s
antihypertensive effects. However, results of current studies contra-
dict each other and therefore our knowledge on the topic is limited.
Oestrogen has been shown to decrease the production of oxidative
stress in the vasculature. Oxidative stress has been linked to high
blood pressure (BP) and therefore its decrease is thought to aid in
prevention of high BP. Excessive vasoconstriction is opposed by nitro-
gen oxide. However, nitrogen oxide production decreases with age
and therefore poses a hypertension risk.
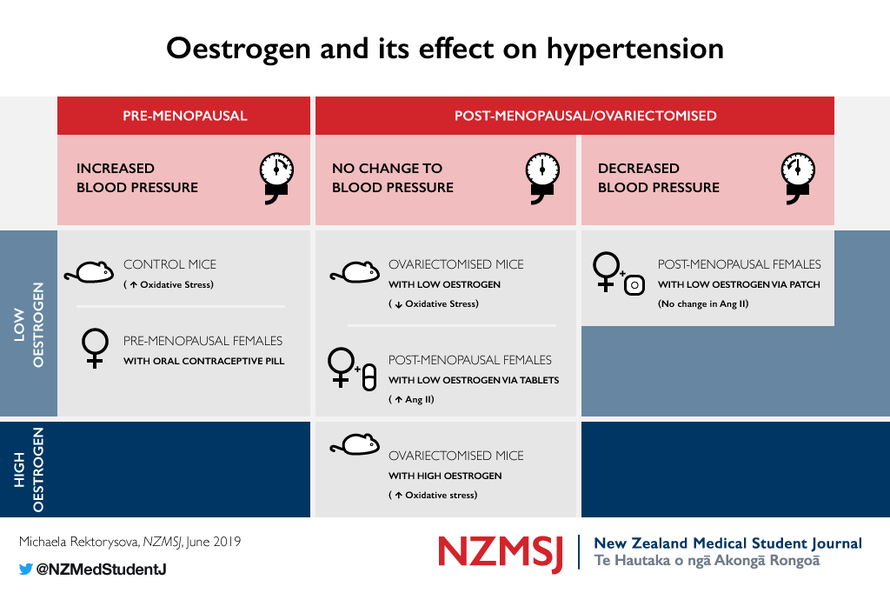
Studies in mice have shown that low doses of oestrogen given to
non-ovariectomised mice have the effect of increasing oxidative
stress. Additionally, high doses of oestrogen in ovariectomised mice
have shown the same effect, however, low doses were shown to
decrease oxidative stress.
Surprisingly, it is shown that the oestrogen in oral contraceptive pills
(OCPs) given to premenopausal women causes an increase in BP. The
effects of hormone-replacement therapy on BP have been shown to
depend on the administration route.
Hypertension
Long-term hypertension is associated with a range of cardiovascular
diseases such as coronary heart disease and stroke. 1 In New Zealand,
hypertension affects 31% of the population. 1 The American Heart
Association reports that on average, more men than women have
high BP, this difference disappears at around 55–64 years of age. 2 The
prevalence of hypertension, regardless of sex, increases with age. 2
Sex is a large determinant in the likelihood of developing hyperten-
sion. 3 It has been found that non-menopausal women are at lower
risk of developing hypertension than men; however, this difference
disappears after menopause. 3 The complexity of hypertension com-
bined with the high prevalence of the condition has led to numerous
attempts to elucidate the pathophysiology of hypertension and its
possible treatments. One of the suggested connections is the link
between the hormone oestrogen, the renin-angiotensin-aldosterone
system (RAAS), and oxidative stress.
Oestrogen
Oestrogen is a sex hormone mainly produced by the ovaries. 4 Its gen-
eral functions include promoting the growth of secondary female sex
characteristics and triggering ovulation. 4 As hypertension prevalence
increases post-menopause, it is suggested that oestrogen, more spe-
cifically the form 17β-oestradiol (E2), provides an antihypertensive
effect on BP that is otherwise lost post-menopause. 4
It has been shown that 17β-oestradiol activates antihypertensive mecha-
nisms such as stimulation of nitrogen oxide (NO) release and a decrease
in oxidative stress, 5 both of which result in relaxation of vascular smooth
muscle, thereby conferring protection from excessive vasoconstriction. 5
The renin-angiotensin-aldosterone system
The RAAS is a mechanism regulating BP and blood volume. 6 It is
activated by a reduction in glomerular filtration rate and one of its
final effectors is angiotensin II (Ang II). Ang II causes vasoconstriction,
production of vasopressin, and the release of aldosterone. All these
actions eventually lead to an increase in water reabsorption, leading
to an increase in BP. 6
The angiotensin II receptor 1 and oxidative stress
One of the effector receptors of Ang II is the angiotensin receptor
type 1 (AT1). 7 AT1 is expressed in many parts of the body, but particu-
larly in vascular smooth muscle cells (VSMC). One of the effects of the
VSMC AT1 receptor is production of reactive oxygen species (ROS). 8
ROS are produced in the Ang II pathway as an intracellular signalling
molecule. Usually, ROS are in balance with antioxidants to prevent
oxidative damage, however, an excess in ROS production results in
an imbalance, termed oxidative stress. 9 Vessel wall oxidative stress
has been found to be involved in the development of hypertension.
AT1 is mainly linked to production of the ROS superoxide anion (O 2 - )
by the nicotinamide adenine dinucleotide phosphate (NADPH) oxi-
dase in the VSMC. NADPH is a part of the electron transport chain
involved in the aerobic production of ATP. 9 ATP is necessary for con-
traction and therefore vasoconstriction. When the vasoconstrictor
Ang II binds to AT1, production of ATP, and therefore activation of
the electron transport chain, will occur. Normally, O 2 , which is creat-
ed as a by-product, would be reduced to water. 9 However, when it is
produced in excessive amounts it can escape the reduction and gain
an electron to become O 2 - . 9
Experimental studies in rodents have shown that Ang II causes an in-
crease in NADPH activity, leading to an excess production of O 2 - . 8 It
has also been shown that O 2 - alone can cause vasoconstriction, which
contributes to the development of hypertension. 10 O 2 - production
can also affect the activity of Ca 2+ and K + ion channels through the
activity of CaMKII, which alters contraction of muscles, adding further
to vasoconstriction 11
Nitrogen oxide
Additional evidence suggests O 2 - interacts with nitrogen oxide (NO).
NO is produced by the endothelium of blood vessels and causes
vasodilation, contributing to the lowering of BP. 5 NO generates per-
oxynitrite (ONOO - ) by reaction with O 2 - . 5 By this action, the amount
of NO is reduced, causing a reduction in its vasodilative effects. 5 But,
as mentioned, ONOO - is created, which can form peroxynirous acid,
a very reactive oxygen species of similar effects as O 2 - . 5 The absence
of NO, and therefore impaired vascular relaxation, is one of the sug-
gested mechanisms for the development of hypertension.
Oestrogen and oxidative stress
Deficiencies in antioxidants have been found in patients suffering
from hypertension. 12 This suggests that not only are ROS increased
in hypertension, but also the concentrations of antioxidants are de-
creased. It has been shown that oxidative stress levels tend to be
higher in males than in females and that when induced by a dose of
Ang II, a larger amount of O 2 - is produced in male arteries than female
arteries. 13,14 This suggests that there is a difference between either
the oxidative stress levels or in the amount of ROS the body can
produce between males and females.
Treatment of ovariectomised rats with E2 has been shown to reduce
the expression of some NADPH regulatory subunits, suggesting that
the production of O 2 - by NADPH can be regulated by E2. 15 Upon
exposure to Ang II, the expression of other NADPH regulatory subu-
nits increases and this can then be normalised by treatment with E2. 16
It has also been found that ovariectomised rats, which cannot pro-
duce their own E2, have an increase in AT1 receptor abundance and
that this effect can be prevented by E2 replacement. 17 This E2-in-
duced AT1 reduction occurs through a decrease of AT1 translation
and a reduction in its binding capacity with Ang II. 17 This suggests that
E2 controls the abundance of AT1 receptors and thereby regulates
Ang II induced production of O 2 - . By decreasing O 2 - production, E2
protects against oxidative stress. As stated above, an increase in ox-
idative stress has been linked to hypertension, but the presence of
oxidative stress does not necessarily lead to hypertension. Unfortu-
nately, this study did not assess the BP of the rats.
Oestrogen dose and blood pressure
A study by Subramanian et al has explored chronic exposure of
non-ovariectomised rats to low levels of E2 and its connection to
hypertension. 18 Rats exposed to 20 ng/day of E2 (low dose) had an
increase in mean arterial pressure compared to controls. In addition,
the E2-treated rats had significantly elevated O 2 - levels.
On the other hand, a study by Meng et al (19) has shown that ova-
riectomised mice do not have a change in BP in response to 20 ng/day
of E2 (low dose). 19 This study also showed that the ovary reduction
itself causes an increase in oxidative stress and that this is reversed by
a low dose of E2. Ovariectomised mice receiving a high dose of E2,
4.2 μg/day, had an increase in oxidative stress in their vasculature and
no significant increase in BP. This is a surprising finding since it con-
flicts with those of many other studies (see above) that demonstrate
how oestrogen leads to a decrease in oxidative stress. The findings of
these authors also suggest a dose-dependent association.
Oestrogen and oral contraceptive pills
Oestrogen is the main ingredient in most oral contraceptive pills
(OCPs). 20 OCPs are taken mainly by non-menopausal women, so it
is supplemental to normal levels and would be comparable to oes-
trogen given to non-ovariectomised mice. A review by Woods et al
has shown that the majority of subjects prescribed OCPs either had
an increase or no change in BP. 21 This is again a surprising finding as
it is conflicts with results of other studies on the anti-oxidative stress
effect of oestrogen.
It is important to consider that OCPs also contains progesterone,
which may confound the effects that are being attributed to oes-
trogen only. The Woods et al article quotes sources supporting the
notion that progesterone has an effect on BP. 22 However, other
evidence suggests that the effect is negligible. 23 Further consistent
research on this topic is required to confirm our understanding of
progesterone and its effect on BP.
Oestrogen and hormone replacement therapy
During menopause, the ovarian production of oestrogen decreases
and the likelihood of hypertension increases. 3 The onset of meno-
pause is accompanied by many symptoms such as insomnia and mi-
graines. Hormone replacement therapy (HRT) is a hormonal sup-
plement aimed at easing the transition from high to low levels of
oestrogen production and to relieve menopausal symptoms. 24 It is
also speculated to have an effect on cardiovascular complications
such as hypertension.
A study by Ichikawa et al explored the effects of transdermal and oral
delivery of low doses of HRT. 24 They found that transdermal delivery
of HRT resulted in a decrease in mean BP, but no change in Ang II
plasma levels. Additionally, oral delivery of HRT did not change BP,
but did increase the Ang II plasma levels. The levels of bradykinin, a
vasodilator, decreased in the transdermal HRT group and increased
in the oral HRT group. The suggested mechanism includes transder-
mal oestrogen activation of NO-mediated relaxation of vasculature.
This leads to downregulation of sympathetic activity, leading to a de-
crease in AT1 messenger ribonucleic acid concentration, leading to
decreased vasoconstriction and oxidative stress. 25
However, oral HRT resulted in an increase in Ang II and bradykinin
levels, but had no effect on BP. It has been suggested that BP did not
change due to the increase in bradykinin alongside Ang II, as their
actions are opposite. Therefore, HRT has varying effects on BP de-
pending on its administration.
It is important to consider that while post-menopausal women do
not produce as much oestrogen as non-menopausal women, they
still produce a small amount. 3 Therefore, post-menopausal women
are not strictly comparable to ovariectomised rodents. This is a lim-
itation in study design that appears to be repeated in most previous
research. A new rodent model, which is comparable to post-meno-
pausal women, is necessary for future research.
Conclusion: the role of oestrogen
In conclusion, the role of oestrogen in hypertension is complex and
not well understood. Studies reviewed in this article have demon-
strated that the addition of oestrogen above its normally produced
levels (i.e. non-ovariectomised rats receiving oestrogen) is linked to
an increase in BP and an increase in oxidative stress. Additionally, it is
noted that giving low doses of oestrogen to ovariectomised rats de-
creases oxidative stress, but that giving high doses increases oxidative
stress. These findings demonstrate that the complexity of oestrogen-
ic action is beyond a simple reduction of oxidative stress effect.
Finally, it is important to recognise that the most valuable studies are
those that include results from humans, as it is ultimately the oes-
trogen received by women in the forms of OCPs and HRT that is of
interest. Further research on the effect of varying doses of oestrogen
in OCPs and HRT is required.
References
1. McLean RM, Williams S, Mann JI, Miller JC, Parnell WR. Blood
pressure and hypertension in New Zealand: results from the 2008/09
Adult Nutrition Survey. NZ Med J. 2013 Apr 5;126(1372):1–4.
2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman
M, et al. Executive summary: heart disease and stroke statistics—2016
update: a report from the American Heart Association. Circulation.
2016 Jan 26;133(4):447–54.
3. Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J
Hypertens. 2011 Jul 1;24(7):740–9.
4. Nelson LR, Bulun SE. Estrogen production and action. J Am Acad
Dermatol. 2001 Sep 1;45(3):S116–24.
5. McIntyre M, Bohr DF, Dominiczak AF. Endothelial function in
hypertension: the role of superoxide anion. Hypertension. 1999
Oct;34(4):539–45.
6. Fu Z, Zhao L, Aylor KW, Carey RM, Barrett EJ, Liu Z.
Angiotensin-(1–7) recruits muscle microvasculature and enhances
insulin’s metabolic action via mas receptor. Hypertension. 2014
Jun;63(6):1219–27.
7. Baker KM, Dostal DE. Angiotensin II stimulation of left ventricular
hypertrophy in adult rat heart: mediation by the AT1 receptor. Am J
Hypertens. 1992 May 1;5(5 Pt 1):276–80.
8. Ebrahimian T, He Y, Schiffrin EL, Touyz RM. Differential regulation
of thioredoxin and NAD (P) H oxidase by angiotensin II in male and
female mice. J Hypertens. 2007 Jun 1;25(6):1263–71.
9. Lee J, Giordano S, Zhang J. Autophagy, mitochondria and
oxidative stress: cross-talk and redox signalling. Biochem J. 2012 Jan
15;441(2):523–40.
10. Zhou MS, Jaimes EA, Raij L. Inhibition of oxidative stress and
improvement of endothelial function by amlodipine in angiotensin
II-infused rats. Am J Hypertens. 2004 Feb 1;17(2):167–71.
11. Sumners CO, Zhu MI, Gelband CH, Posner P. Angiotensin II type 1
receptor modulation of neuronal K+ and Ca2+ currents: intracellular
mechanisms. Am J Physiol. 1996 Jul 1;271(1):C154–63.
12. Chaves FJ, Mansego ML, Blesa S, Gonzalez-Albert V, Jiménez
J, Tormos MC, et al. Inadequate cytoplasmic antioxidant enzymes
response contributes to the oxidative stress in human hypertension.
Am J Hypertens. 2007 Jan 1;20(1):62–9.
13. Lacy F, Kailasam MT, O’connor DT, Schmid-Schönbein GW,
Parmer RJ. Plasma hydrogen peroxide production in human essential
hypertension: role of heredity, gender, and ethnicity. Hypertension.
2000 Nov;36(5):878–84.
14. De Silva TM, Broughton BR, Drummond GR, Sobey CG, Miller
AA. Gender influences cerebral vascular responses to angiotensin II
through Nox2-derived reactive oxygen species. Stroke. 2009 Apr
1;40(4):1091–7.
15. Arias-Loza PA, Muehlfelder M, Pelzer T. Estrogen and estrogen
receptors in cardiovascular oxidative stress. Pflugers Arch. 2013 May
1;465(5):739–46.
16. Bhatia K, Elmarakby AA, El-Remessey A, Sullivan JC. Oxidative
stress contributes to sex differences in angiotensin II-mediated
hypertension in spontaneously hypertensive rats. Am J Physiol
Regul Integr Comp Physio. 2012 Jan 1;302(2). DOI:10.1152/
ajpregu.00546.201
17. Wu Z, Zheng W, Sandberg K. Estrogen regulates adrenal
angiotensin type 1 receptors by modulating adrenal angiotensin levels.
Endocrinology. 2003 Apr 1;144(4):1350–6.
18. Subramanian M, Balasubramanian P, Garver H, Northcott C,
Zhao H, Haywood JR, Fink GD, MohanKumar SM, MohanKumar PS.
Chronic estradiol-17β exposure increases superoxide production in
the rostral ventrolateral medulla and causes hypertension: reversal
by resveratrol. Am J Physiol Regul Integr Comp Physiol. 2011 Mar
16;300(6):R1560–8. DOI:10.1152/ajpregu.00020.2011.
19. Meng X, Dai X, Liao TD, D’ambrosio M, Wang F, Yang JJ, et al.
Dose-dependent toxic effects of high-dose estrogen on renal and
cardiac injury in surgically postmenopausal mice. Life Sci. 2011 Jan
17;88(3–4):178–86.
20. Genazzani AR, Mannella P, Simoncini T. Drospirenone and its
antialdosterone properties. Climacteric. 2007 Jan 1;10(Suppl 1):11–8.
21. Woods JW. Oral contraceptives and hypertension. Hypertension.
1988 Mar;11(3 pt 2):II11–5.
22. Weir RJ. Effect on blood pressure of changing from high to low
dose steroid preparations in women with oral contraceptive induced
hypertension. Scott Med J. 1982 Jul;27(3):212–5.
23. Hussain SF. Progestogen-only pills and high blood pressure: is
there an association?: a literature review. Contraception. 2004 Feb
1;69(2):89–97.
24. Ichikawa J, Sumino H, Ichikawa S, Ozaki M. Different effects of
transdermal and oral hormone replacement therapy on the renin-
angiotensin system, plasma bradykinin level, and blood pressure of
normotensive postmenopausal women. Am J Hypertens. 2006 Jul
1;19(7):744–9.
25. Dean SA, Tan J, O’Brien ER, Leenen FH. 17β-Estradiol
downregulates tissue angiotensin-converting enzyme and ANG
II type 1 receptor in female rats. Am J Physiol Regul Integr Comp
Physiol. 2005 Mar;288(3):R759–66.
Conflicts of Interest
Michaela is a student reviewer for the NZMSJ. This article has gone
through a double-blinded peer review process applied to all articles
submitted to the NZMSJ, and has been accepted after achieving the
standards required for publication. The author has no other conflict
of interest.
Correspondence
Michaela Rektorysova: michaela.rektorysova@gmail.com
Michaela Rektorysova
School of Medicine
Faculty of Medical and Health Sciences
University of Auckland
Abstract
Non-menopausal women have a lower incidence of hypertension and
cardiovascular complications compared to age-matched men. This
cardiovascular advantage is thought to be the result of oestrogen’s
antihypertensive effects. However, results of current studies contra-
dict each other and therefore our knowledge on the topic is limited.
Oestrogen has been shown to decrease the production of oxidative
stress in the vasculature. Oxidative stress has been linked to high
blood pressure (BP) and therefore its decrease is thought to aid in
prevention of high BP. Excessive vasoconstriction is opposed by nitro-
gen oxide. However, nitrogen oxide production decreases with age
and therefore poses a hypertension risk.
Studies in mice have shown that low doses of oestrogen given to
non-ovariectomised mice have the effect of increasing oxidative
stress. Additionally, high doses of oestrogen in ovariectomised mice
have shown the same effect, however, low doses were shown to
decrease oxidative stress.
Surprisingly, it is shown that the oestrogen in oral contraceptive pills
(OCPs) given to premenopausal women causes an increase in BP. The
effects of hormone-replacement therapy on BP have been shown to
depend on the administration route.
Hypertension
Long-term hypertension is associated with a range of cardiovascular
diseases such as coronary heart disease and stroke. 1 In New Zealand,
hypertension affects 31% of the population. 1 The American Heart
Association reports that on average, more men than women have
high BP, this difference disappears at around 55–64 years of age. 2 The
prevalence of hypertension, regardless of sex, increases with age. 2
Sex is a large determinant in the likelihood of developing hyperten-
sion. 3 It has been found that non-menopausal women are at lower
risk of developing hypertension than men; however, this difference
disappears after menopause. 3 The complexity of hypertension com-
bined with the high prevalence of the condition has led to numerous
attempts to elucidate the pathophysiology of hypertension and its
possible treatments. One of the suggested connections is the link
between the hormone oestrogen, the renin-angiotensin-aldosterone
system (RAAS), and oxidative stress.
Oestrogen
Oestrogen is a sex hormone mainly produced by the ovaries. 4 Its gen-
eral functions include promoting the growth of secondary female sex
characteristics and triggering ovulation. 4 As hypertension prevalence
increases post-menopause, it is suggested that oestrogen, more spe-
cifically the form 17β-oestradiol (E2), provides an antihypertensive
effect on BP that is otherwise lost post-menopause. 4
It has been shown that 17β-oestradiol activates antihypertensive mecha-
nisms such as stimulation of nitrogen oxide (NO) release and a decrease
in oxidative stress, 5 both of which result in relaxation of vascular smooth
muscle, thereby conferring protection from excessive vasoconstriction. 5
The renin-angiotensin-aldosterone system
The RAAS is a mechanism regulating BP and blood volume. 6 It is
activated by a reduction in glomerular filtration rate and one of its
final effectors is angiotensin II (Ang II). Ang II causes vasoconstriction,
production of vasopressin, and the release of aldosterone. All these
actions eventually lead to an increase in water reabsorption, leading
to an increase in BP. 6
The angiotensin II receptor 1 and oxidative stress
One of the effector receptors of Ang II is the angiotensin receptor
type 1 (AT1). 7 AT1 is expressed in many parts of the body, but particu-
larly in vascular smooth muscle cells (VSMC). One of the effects of the
VSMC AT1 receptor is production of reactive oxygen species (ROS). 8
ROS are produced in the Ang II pathway as an intracellular signalling
molecule. Usually, ROS are in balance with antioxidants to prevent
oxidative damage, however, an excess in ROS production results in
an imbalance, termed oxidative stress. 9 Vessel wall oxidative stress
has been found to be involved in the development of hypertension.
AT1 is mainly linked to production of the ROS superoxide anion (O 2 - )
by the nicotinamide adenine dinucleotide phosphate (NADPH) oxi-
dase in the VSMC. NADPH is a part of the electron transport chain
involved in the aerobic production of ATP. 9 ATP is necessary for con-
traction and therefore vasoconstriction. When the vasoconstrictor
Ang II binds to AT1, production of ATP, and therefore activation of
the electron transport chain, will occur. Normally, O 2 , which is creat-
ed as a by-product, would be reduced to water. 9 However, when it is
produced in excessive amounts it can escape the reduction and gain
an electron to become O 2 - . 9
Experimental studies in rodents have shown that Ang II causes an in-
crease in NADPH activity, leading to an excess production of O 2 - . 8 It
has also been shown that O 2 - alone can cause vasoconstriction, which
contributes to the development of hypertension. 10 O 2 - production
can also affect the activity of Ca 2+ and K + ion channels through the
activity of CaMKII, which alters contraction of muscles, adding further
to vasoconstriction 11
Nitrogen oxide
Additional evidence suggests O 2 - interacts with nitrogen oxide (NO).
NO is produced by the endothelium of blood vessels and causes
vasodilation, contributing to the lowering of BP. 5 NO generates per-
oxynitrite (ONOO - ) by reaction with O 2 - . 5 By this action, the amount
of NO is reduced, causing a reduction in its vasodilative effects. 5 But,
as mentioned, ONOO - is created, which can form peroxynirous acid,
a very reactive oxygen species of similar effects as O 2 - . 5 The absence
of NO, and therefore impaired vascular relaxation, is one of the sug-
gested mechanisms for the development of hypertension.
Oestrogen and oxidative stress
Deficiencies in antioxidants have been found in patients suffering
from hypertension. 12 This suggests that not only are ROS increased
in hypertension, but also the concentrations of antioxidants are de-
creased. It has been shown that oxidative stress levels tend to be
higher in males than in females and that when induced by a dose of
Ang II, a larger amount of O 2 - is produced in male arteries than female
arteries. 13,14 This suggests that there is a difference between either
the oxidative stress levels or in the amount of ROS the body can
produce between males and females.
Treatment of ovariectomised rats with E2 has been shown to reduce
the expression of some NADPH regulatory subunits, suggesting that
the production of O 2 - by NADPH can be regulated by E2. 15 Upon
exposure to Ang II, the expression of other NADPH regulatory subu-
nits increases and this can then be normalised by treatment with E2. 16
It has also been found that ovariectomised rats, which cannot pro-
duce their own E2, have an increase in AT1 receptor abundance and
that this effect can be prevented by E2 replacement. 17 This E2-in-
duced AT1 reduction occurs through a decrease of AT1 translation
and a reduction in its binding capacity with Ang II. 17 This suggests that
E2 controls the abundance of AT1 receptors and thereby regulates
Ang II induced production of O 2 - . By decreasing O 2 - production, E2
protects against oxidative stress. As stated above, an increase in ox-
idative stress has been linked to hypertension, but the presence of
oxidative stress does not necessarily lead to hypertension. Unfortu-
nately, this study did not assess the BP of the rats.
Oestrogen dose and blood pressure
A study by Subramanian et al has explored chronic exposure of
non-ovariectomised rats to low levels of E2 and its connection to
hypertension. 18 Rats exposed to 20 ng/day of E2 (low dose) had an
increase in mean arterial pressure compared to controls. In addition,
the E2-treated rats had significantly elevated O 2 - levels.
On the other hand, a study by Meng et al (19) has shown that ova-
riectomised mice do not have a change in BP in response to 20 ng/day
of E2 (low dose). 19 This study also showed that the ovary reduction
itself causes an increase in oxidative stress and that this is reversed by
a low dose of E2. Ovariectomised mice receiving a high dose of E2,
4.2 μg/day, had an increase in oxidative stress in their vasculature and
no significant increase in BP. This is a surprising finding since it con-
flicts with those of many other studies (see above) that demonstrate
how oestrogen leads to a decrease in oxidative stress. The findings of
these authors also suggest a dose-dependent association.
Oestrogen and oral contraceptive pills
Oestrogen is the main ingredient in most oral contraceptive pills
(OCPs). 20 OCPs are taken mainly by non-menopausal women, so it
is supplemental to normal levels and would be comparable to oes-
trogen given to non-ovariectomised mice. A review by Woods et al
has shown that the majority of subjects prescribed OCPs either had
an increase or no change in BP. 21 This is again a surprising finding as
it is conflicts with results of other studies on the anti-oxidative stress
effect of oestrogen.
It is important to consider that OCPs also contains progesterone,
which may confound the effects that are being attributed to oes-
trogen only. The Woods et al article quotes sources supporting the
notion that progesterone has an effect on BP. 22 However, other
evidence suggests that the effect is negligible. 23 Further consistent
research on this topic is required to confirm our understanding of
progesterone and its effect on BP.
Oestrogen and hormone replacement therapy
During menopause, the ovarian production of oestrogen decreases
and the likelihood of hypertension increases. 3 The onset of meno-
pause is accompanied by many symptoms such as insomnia and mi-
graines. Hormone replacement therapy (HRT) is a hormonal sup-
plement aimed at easing the transition from high to low levels of
oestrogen production and to relieve menopausal symptoms. 24 It is
also speculated to have an effect on cardiovascular complications
such as hypertension.
A study by Ichikawa et al explored the effects of transdermal and oral
delivery of low doses of HRT. 24 They found that transdermal delivery
of HRT resulted in a decrease in mean BP, but no change in Ang II
plasma levels. Additionally, oral delivery of HRT did not change BP,
but did increase the Ang II plasma levels. The levels of bradykinin, a
vasodilator, decreased in the transdermal HRT group and increased
in the oral HRT group. The suggested mechanism includes transder-
mal oestrogen activation of NO-mediated relaxation of vasculature.
This leads to downregulation of sympathetic activity, leading to a de-
crease in AT1 messenger ribonucleic acid concentration, leading to
decreased vasoconstriction and oxidative stress. 25
However, oral HRT resulted in an increase in Ang II and bradykinin
levels, but had no effect on BP. It has been suggested that BP did not
change due to the increase in bradykinin alongside Ang II, as their
actions are opposite. Therefore, HRT has varying effects on BP de-
pending on its administration.
It is important to consider that while post-menopausal women do
not produce as much oestrogen as non-menopausal women, they
still produce a small amount. 3 Therefore, post-menopausal women
are not strictly comparable to ovariectomised rodents. This is a lim-
itation in study design that appears to be repeated in most previous
research. A new rodent model, which is comparable to post-meno-
pausal women, is necessary for future research.
Conclusion: the role of oestrogen
In conclusion, the role of oestrogen in hypertension is complex and
not well understood. Studies reviewed in this article have demon-
strated that the addition of oestrogen above its normally produced
levels (i.e. non-ovariectomised rats receiving oestrogen) is linked to
an increase in BP and an increase in oxidative stress. Additionally, it is
noted that giving low doses of oestrogen to ovariectomised rats de-
creases oxidative stress, but that giving high doses increases oxidative
stress. These findings demonstrate that the complexity of oestrogen-
ic action is beyond a simple reduction of oxidative stress effect.
Finally, it is important to recognise that the most valuable studies are
those that include results from humans, as it is ultimately the oes-
trogen received by women in the forms of OCPs and HRT that is of
interest. Further research on the effect of varying doses of oestrogen
in OCPs and HRT is required.
References
1. McLean RM, Williams S, Mann JI, Miller JC, Parnell WR. Blood
pressure and hypertension in New Zealand: results from the 2008/09
Adult Nutrition Survey. NZ Med J. 2013 Apr 5;126(1372):1–4.
2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman
M, et al. Executive summary: heart disease and stroke statistics—2016
update: a report from the American Heart Association. Circulation.
2016 Jan 26;133(4):447–54.
3. Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J
Hypertens. 2011 Jul 1;24(7):740–9.
4. Nelson LR, Bulun SE. Estrogen production and action. J Am Acad
Dermatol. 2001 Sep 1;45(3):S116–24.
5. McIntyre M, Bohr DF, Dominiczak AF. Endothelial function in
hypertension: the role of superoxide anion. Hypertension. 1999
Oct;34(4):539–45.
6. Fu Z, Zhao L, Aylor KW, Carey RM, Barrett EJ, Liu Z.
Angiotensin-(1–7) recruits muscle microvasculature and enhances
insulin’s metabolic action via mas receptor. Hypertension. 2014
Jun;63(6):1219–27.
7. Baker KM, Dostal DE. Angiotensin II stimulation of left ventricular
hypertrophy in adult rat heart: mediation by the AT1 receptor. Am J
Hypertens. 1992 May 1;5(5 Pt 1):276–80.
8. Ebrahimian T, He Y, Schiffrin EL, Touyz RM. Differential regulation
of thioredoxin and NAD (P) H oxidase by angiotensin II in male and
female mice. J Hypertens. 2007 Jun 1;25(6):1263–71.
9. Lee J, Giordano S, Zhang J. Autophagy, mitochondria and
oxidative stress: cross-talk and redox signalling. Biochem J. 2012 Jan
15;441(2):523–40.
10. Zhou MS, Jaimes EA, Raij L. Inhibition of oxidative stress and
improvement of endothelial function by amlodipine in angiotensin
II-infused rats. Am J Hypertens. 2004 Feb 1;17(2):167–71.
11. Sumners CO, Zhu MI, Gelband CH, Posner P. Angiotensin II type 1
receptor modulation of neuronal K+ and Ca2+ currents: intracellular
mechanisms. Am J Physiol. 1996 Jul 1;271(1):C154–63.
12. Chaves FJ, Mansego ML, Blesa S, Gonzalez-Albert V, Jiménez
J, Tormos MC, et al. Inadequate cytoplasmic antioxidant enzymes
response contributes to the oxidative stress in human hypertension.
Am J Hypertens. 2007 Jan 1;20(1):62–9.
13. Lacy F, Kailasam MT, O’connor DT, Schmid-Schönbein GW,
Parmer RJ. Plasma hydrogen peroxide production in human essential
hypertension: role of heredity, gender, and ethnicity. Hypertension.
2000 Nov;36(5):878–84.
14. De Silva TM, Broughton BR, Drummond GR, Sobey CG, Miller
AA. Gender influences cerebral vascular responses to angiotensin II
through Nox2-derived reactive oxygen species. Stroke. 2009 Apr
1;40(4):1091–7.
15. Arias-Loza PA, Muehlfelder M, Pelzer T. Estrogen and estrogen
receptors in cardiovascular oxidative stress. Pflugers Arch. 2013 May
1;465(5):739–46.
16. Bhatia K, Elmarakby AA, El-Remessey A, Sullivan JC. Oxidative
stress contributes to sex differences in angiotensin II-mediated
hypertension in spontaneously hypertensive rats. Am J Physiol
Regul Integr Comp Physio. 2012 Jan 1;302(2). DOI:10.1152/
ajpregu.00546.201
17. Wu Z, Zheng W, Sandberg K. Estrogen regulates adrenal
angiotensin type 1 receptors by modulating adrenal angiotensin levels.
Endocrinology. 2003 Apr 1;144(4):1350–6.
18. Subramanian M, Balasubramanian P, Garver H, Northcott C,
Zhao H, Haywood JR, Fink GD, MohanKumar SM, MohanKumar PS.
Chronic estradiol-17β exposure increases superoxide production in
the rostral ventrolateral medulla and causes hypertension: reversal
by resveratrol. Am J Physiol Regul Integr Comp Physiol. 2011 Mar
16;300(6):R1560–8. DOI:10.1152/ajpregu.00020.2011.
19. Meng X, Dai X, Liao TD, D’ambrosio M, Wang F, Yang JJ, et al.
Dose-dependent toxic effects of high-dose estrogen on renal and
cardiac injury in surgically postmenopausal mice. Life Sci. 2011 Jan
17;88(3–4):178–86.
20. Genazzani AR, Mannella P, Simoncini T. Drospirenone and its
antialdosterone properties. Climacteric. 2007 Jan 1;10(Suppl 1):11–8.
21. Woods JW. Oral contraceptives and hypertension. Hypertension.
1988 Mar;11(3 pt 2):II11–5.
22. Weir RJ. Effect on blood pressure of changing from high to low
dose steroid preparations in women with oral contraceptive induced
hypertension. Scott Med J. 1982 Jul;27(3):212–5.
23. Hussain SF. Progestogen-only pills and high blood pressure: is
there an association?: a literature review. Contraception. 2004 Feb
1;69(2):89–97.
24. Ichikawa J, Sumino H, Ichikawa S, Ozaki M. Different effects of
transdermal and oral hormone replacement therapy on the renin-
angiotensin system, plasma bradykinin level, and blood pressure of
normotensive postmenopausal women. Am J Hypertens. 2006 Jul
1;19(7):744–9.
25. Dean SA, Tan J, O’Brien ER, Leenen FH. 17β-Estradiol
downregulates tissue angiotensin-converting enzyme and ANG
II type 1 receptor in female rats. Am J Physiol Regul Integr Comp
Physiol. 2005 Mar;288(3):R759–66.
Conflicts of Interest
Michaela is a student reviewer for the NZMSJ. This article has gone
through a double-blinded peer review process applied to all articles
submitted to the NZMSJ, and has been accepted after achieving the
standards required for publication. The author has no other conflict
of interest.
Correspondence
Michaela Rektorysova: michaela.rektorysova@gmail.com