Download PDF
Dr Paul Huggan
BSc (Hons) MBChB FRACP FRCP (Edin)
Clinical Director at the Department of Infectious Disease
Waikato Hospital and District Health Board
Abstract
Sepsis is defined as a life-threatening disorder caused by a dysregulated immune response to infection. It can be a challenging condition to recognise, and patients with sepsis are some of the most complex in any medical or surgical service. Sepsis patients are demanding due to their illness severity and underlying morbidities. Successful resuscitation relies on recognition, early delivery of appropriate anti-microbial therapy and management of shock/organ failure. Parallel efforts are required to identify and treat the infection driving the sepsis response, often leading to multiple investigations, procedures, and long stays in hospital. Ongoing efforts are required to recognise and treat sepsis as the population vulnerabilities that drive infection persist in New Zealand.
Introduction
Infectious diseases are the most common cause of hospitalisation in New Zealand (1). Rates of infection-related hospitalisation have been increasing for several decades (1). Infectious diseases and sepsis are more common amongst older adults, Māori and Pacific people, and those living in conditions of high socio-economic deprivation (2). These are independent risks – for example, non-Māori people living in settings of deprivation are also at increased risk (3). Sepsis presentations mirror infectious disease epidemiology – where there is more infection in a region or population, there is more sepsis (2).
Presentations with sepsis present to every sub-specialty of medicine or surgery. Because every specialty deals with some sepsis, counting cases has to be done using administrative data. In a study of sepsis epidemiology in the Waikato region, we took patients with primary admission codes for major infectious diseases, then identified the sickest patients in this group by looking for secondary codes describing organ failure. We found that 8% of all admissions over a five year period had a primary infectious disease code, of which 10% had organ failure codes (2). That means that about 1% of all admissions met this definition of sepsis. The 28-day mortality in this group was almost 19%, increasing to 37% in patients admitted to our intensive care unit (ICU).
Case vignette one
Mr M, a 61-year-old man, has been admitted to hospital for management of hemiparetic stroke. He has benign prostatic hypertrophy and was catheterised two days ago for new urinary retention. You are asked to see him with a fever of 38.50C. He has rigors and is confused. You find his peripheries are warm, pulse 115 beats per minute, respiratory rate 28 breaths per minute, systolic blood pressure (SBP) 110 (normal SBP in hospital has been 160), SpO2 95% on room air. He has no signs of peripheral line infection. There are no abnormalities on auscultation of the chest. He has a soft abdomen and a small amount of concentrated urine is in the catheter bag.
-------------------------------------------------------------------------------------------------------------------------------------
Box 1. Definitions of sepsis and related terms.
Sepsis 3.0 narrative definition (proposed in the Third International Consensus Definitions for sepsis and septic shock) (4):
“Sepsis is characterised by a life-threatening organ dysfunction due to a dysregulated host response to infection.”
Lay narrative definitions of sepsis:
“An acute change in total Sequential Organ Failure Assessment Score (SOFA) ≥2 points consequent to infection.”
Septicaemia:
An outdated (but commonly recognised) term indicating a bloodborne inflammatory response due to infection.
Bacteraemia:
The presence of bacteria in the blood.
Hypoperfusion:
Reduced flow of blood through tissue.
Hypotension (in adults):
A severe variant of sepsis that has an in-hospital mortality in excess of 40%.
Narrative definition (4): “a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality.”
Clinical definition (4): “persisting hypotension requiring vasopressors to maintain MAP ≥65 mmHg plus a serum lactate level >2 mmol/L despite adequate volume resuscitation.”
--------------------------------------------------------------------------------------------------------------------------------------
What is sepsis and how is it recognised?
Current definitions of sepsis, septic shock and related terms are given in Box 1.
Biologically, sepsis is best conceived as an acute inflammatory disease of the circulation caused by infection, or “malignant intravascular inflammation” (5). Disturbance of endothelial and circulatory function (further described below) leads to tissue injury, which accumulates and progresses over time. This is why the earliest signs of sepsis can be non-specific, but then evolve quickly. Presentations can vary depending on the patient, the stage of the illness at presentation, the nature of the underlying infection, variable manifestations of organ failure, and, to a certain extent, the micro-organism involved.
If sepsis-related tissue injury goes untreated (i.e. with antimicrobials, management of the infection, and careful supportive care) it gets harder to manage as time passes, while at the same time, the underlying infection becomes increasingly obvious. This is literally a classic medical dilemma, described succinctly by Niccolo Machiavelli in ‘The Prince’, written in 1532:
"As the physicians say it happens in hectic fever, that in the beginning of the malady it is easy to cure but difficult to detect, but in the course of time, not having been either detected or treated in the beginning, it becomes easy to detect but difficult to cure."
Our patient Mr M appears very unwell and sepsis seems likely, but the underlying infection has not yet been identified. At this stage, the formal “Sepsis 3.0” (see Box 1) definition of sepsis cannot be applied, as this requires laboratory testing. Guidelines published in New Zealand by the Best Practice Advocacy Centre (BPAC) recommend the use of available findings to categorise patients as being at a high, moderate, or low risk of sepsis (6). The New Zealand Sepsis Trust (NZST) has collaborated with the UK Sepsis Trust (UKST) to publish a Sepsis Screening and Action Tool (Figure 1), which simplifies the assessment process. It is available at www.sepsis.org.nz and uses the presence of Red Flags to justify rapid investigation and treatment. Red Flags cannot easily be memorised, but note that in non-pregnant adults, any single vital sign that would score a 3 using the national early warning score (EWS) counts as a Red Flag. Paediatric and maternal physiology differ, which is why there are specific tools for these groups (available at www.sepsis.org.nz). Some patients with less severe abnormalities (Amber Flags) are still at moderate risk of progressing to sepsis and need careful assessment.
Mr M has several Red and Amber Flags. He has new onset confusion, his nurse is worried about his condition, he has dropped his SBP greater (40 mmHg below his normal), and he has a respiratory rate of 28 breaths per minute. He needs immediate investigation and treatment using “Sepsis Six” (Figure 2). This is printed on the reverse of the Sepsis Screening and Action Tool, focusing attention on securing a diagnosis and administering effective antibiotics.
Case vignette two
The house officer and nurse both attended sepsis training as part of their hospital orientation. They use the screening and action tool to confirm Red Flag sepsis. The house officer checks the local antimicrobial guideline for sepsis. Because of recent catheterisation in a male, and in the absence of any other clinical signs, she looks for recommendations for treating sepsis of urinary source. Ceftriaxone and gentamicin are recommended. She asks the nurse to prepare these while she inserts a new intravenous line, drawing blood for culture and serum lactate. While antibiotics are being given, the blood pressure is re-checked and confirmed at 105 systolic. The house surgeon decides to give a 500 mL bolus of normal saline over 30 minutes. As the SpO2 is 95% on room air, she decides not to give oxygen, even though the patient’s respiratory rate is elevated. The lab calls a few minutes later to say that the serum lactate is 3.1 mmol/litre (normal range 0.5–2.0).
What are the principles of caring for a patient with sepsis?
Mr M has received prompt treatment with appropriate antibiotics and is being given a fluid bolus. A likely source has been identified and the outlook might be good. However, at this stage it is not clear whether the diagnosis of a urinary tract infection is correct, or indeed whether Mr M will respond appropriately. He needs to be carefully monitored with two things in mind:
The need to keep asking the first question is self-explanatory. The second question needs a little more explanation. The whole immune system is involved in the sepsis response, which is expressed clinically through changes in endothelial and circulatory function. Increased endothelial permeability, neutrophil diapodesis, release of nitric oxide, and migration of inflammatory cells into the interstitium cause tissue oedema and cell damage. When this happens in the skin, the end result is the swelling, redness, heat, and pain (tumor et rubor cum calor et dolore) that can be seen with the naked eye. In sepsis, these changes occur in every major organ, causing tissue damage while also reducing effective circulatory volume (preload) and peripheral resistance (afterload). Damage to the microvasculature combined with low cardiac output leads to global tissue hypoxia. This triggers an increase in production of lactic acid, which in the case of Mr M was elevated above 2 mmol/litre after resuscitation, providing an additional Red Flag.
Available signs of global tissue hypoxia include hypotension, elevated lactate, tachypnoea, and reduced level of consciousness (patient is unresponsive or responds only to voice or pain). If any of these are present at initiation of the Sepsis Six, the patient should be assessed regularly to ensure that things improve. Typically, this means seeing the patient after two to four hours have passed, or earlier if there is reason for concern. NZST have published a Sepsis Hypoperfusion Pathway to aid decision making in this setting. As sepsis is much more common amongst elderly patients, end-of-life decision making may come in to play. However, it is important to recognise that persistent hypoperfusion is a precursor to a poor outcome. Regardless of the underlying situation, involving a senior decision maker or intensive care/medical emergency team is almost always appropriate, even if eligibility for intensive therapies is in doubt.
Case vignette three
Despite a 500 mL fluid bolus, Mr M’s blood pressure continued to fall (to 100 systolic) and his respiratory rate increased to 35 breaths per minute with an SpO2 of 91%. Oxygen was administered via mask and the SpO2 improved to 95%. A further 500 mL fluid bolus was administered but the blood pressure remains unchanged, leading to administration of a 1000 mL fluid bolus over the second hour. By this stage, Mr M has had 20 mL/kg of chrytalloid fluid, but remains drowsy and tachypnoeic. The EWS increases to 10. Despite concerns that the underlying stroke may limit Mr M’s eligibility for intensive care admission, the house surgeon decides to call her registrar, who advises requesting a further lactate and requesting an intensive care review. The intensive care team identify that the blood pressure has fallen further to 90 systolic, there has been no urine produced for four hours and the lactate remains elevated at 4.1 mmol/l. A decision is made to initiate vasopressor support and transfer to the high dependency unit (HDU). The stroke unit consultant attends and confirms that resuscitation efforts are appropriate.
Were the antibiotics appropriate?
Now the situation is looking very serious. Mr M satisfies the definition of septic shock given in Box 1, increasing his risk of in-hospital death to 40%. From the point of view of his antimicrobial therapy, it might be tempting to continue adding “broad spectrum” antibiotics into the mix. However, having sustained a serious inflammatory injury, the body’s response to sepsis can persist in the first few hours, despite effective therapy. This will be particularly true if an abscess, perforation, or other serious anatomical lesion hasn’t been identified and correctly treated (a popular rule of thumb is to “never go to sleep on pus”). With that caveat in mind, if the first clinical assessment is felt to be accurate and antibiotics have been given using an accepted local guideline, it is reasonable to observe response over several hours before considering additional agents. At our hospital, 20% of patients admitted to the ICU or HDU with clinical sepsis have no clearly identified source, but the antibiotics recommended in our guidelines are active in over 95% of cases.
Consider imaging or direct intervention if there might be a drainable collection. Follow the microbiology results carefully. Once susceptibility results are available a decision can be made about how long to continue antibiotic treatment and whether to use an intravenous or oral route.
Clinical vignette four
Mr M spent some time on oxygen and phenylephrine while additional fluid was administered to a total of 30 ml/kg. A chest x-ray showed no pulmonary parenchymal changes. The EWS slowly improved and phenylephrine was weaned overnight. A gram-negative bacillus was identified in blood after six hours of incubation. By the following afternoon this was reported as an Escherichia coli (E.coli), and the same organism was found in a catheter urine specimen, confirming a diagnosis of catheter-associated urinary tract infection. Ceftriaxone was continued for 48 hours, but by the end of the second day, the E.coli was found to be susceptible to amoxicillin. After insertion of a nasogastric tube, ceftriaxone was stopped and amoxicillin given enterally. Mr M improved quickly and a decision was made to stop antibiotics after seven days. He was transferred back to the stroke unit. The same house surgeon met with the family and was surprised to find that none of them had ever heard of sepsis. As Mr M is going to be discharged with a urinary catheter, his family ask how they would spot sepsis if it happened at home.
How do I talk to patients and families about sepsis?
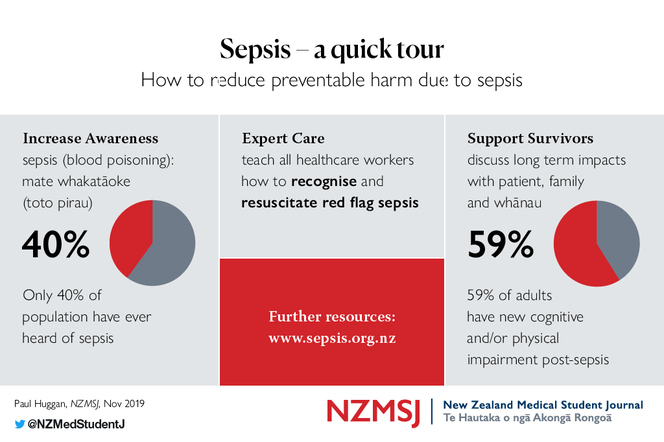
NZST volunteers surveyed almost 300 people attending the 2019 Fieldays event, and found that only 40% had even heard of the term “sepsis”. Other evidence suggests that these problems of awareness and understanding extend to health care workers, who can struggle to explain sepsis to patients and families. This means that patients often leave hospital with an incomplete understanding of their illness and its consequences. We recommend describing sepsis as “blood poisoning (toto pirau) due to infection”, whether or not people have heard of sepsis. This speaks simply to the severity and pathophysiology of the condition, and provides people with a description they can easily remember and share with others. Many people will have heard of the term “septicaemia”, which was in common use in the medical profession until the early 1990s. It is worth explaining that the preferred term for septicaemia is sepsis.
NZST has produced an information leaflet in English and Te Reo Māori for patients, family, and whānau, available on the health navigator website (search sepsis) and at www.sepsis.org.nz.
What are the unique challenges in managing sepsis in New Zealand?
In the Waikato study, the incidence of sepsis amongst Māori was three times higher than amongst non-Māori. Sepsis incidence is one of a range of health indicators that are worse for Māori than non-Māori (2). This inequality (between group difference in health status) has its origins in inequity (defined as maldistribution of resource), which is a manifestation of institutionalised, interpersonal, and internalised racism (7). Inequity and racism contribute to overcrowding, malnutrition, smoking rates, skin and dental disease, poor health literacy, and difficulty accessing and relating to health care providers.
These, and other factors, not only predispose to infection, but also to serious morbidities that are frequently complicated by infection, for example cancer and rheumatic fever. Sepsis occurs as a complication of infection, ergo, any population with higher rates of infection will have higher rates of sepsis. It would follow that the burdens of sepsis amongst Māori will not be lessened until entrenched health and socio-economic inequities are addressed.
What can I do to prevent sepsis or help those who are recovering from it?
Some deaths from sepsis are preventable with early recognition and response. That is why the mission of the New Zealand Sepsis Trust is to ensure that there are “no preventable deaths due to sepsis in New Zealand”. Significant resources are already devoted to the prevention of infection. Examples include vaccination, sanitation, hand and procedural hygiene, and hospital infection control programs. We also support antimicrobial stewardship efforts. Antimicrobial stewardship programs aim to restrict total antimicrobial use while improving antimicrobial prescribing decisions. While it will always remain important to minimise use of antibiotics in low-risk settings, suspected sepsis is the paramount condition in which early guideline-driven antimicrobial therapy is appropriate.
The NZST’s goals are to ensure: i) that all New Zealanders understand sepsis is deadly, but preventable and treatable; ii) that standardised emergency treatment is provided by clinicians with access to high quality sepsis education; and iii) that sepsis survivors and their families receive support and education to aid their recovery. Sepsis survivors are key partners in our work, helping us to understand the longer term impacts of sepsis on day-to-day physical and cognitive function. For a condition that has twice the mortality of myocardial infarction or stroke, remarkably little has been done to elevate sepsis as a major health concern. You can take part in changing this and find out more at www.sepsis.org.nz.
In summary sepsis is a deadly condition amenable to improved outcomes through increased community awareness, standardised emergency treatment, and efforts to prevent and treat infection in the community.
References
About the author
Dr Paul Huggan, BSc (Hons) MBChB FRACP FRCP (Edin). is Clinical Director, at the Department of Infectious Disease, Waikato Hospital and District Health Board.
Paul trained in acute medicine and infectious diseases in the UK, New Zealand and Singapore before taking up a position in the Waikato. He has a career interest in improving care for severe infection across the spectrum of acute care, leading to the foundation of the New Zealand Sepsis Trust, an organisation dedicated to improving sepsis awareness, clinical care and survivor support.
Acknowledgements
The author would like to thank Dr Katie Walland for reviewing the manuscript prior to submission.
Conflicts of interest
Co-founder and Chair, Sepsis Trust NZ.
Correspondence
Dr Paul Huggan: [email protected]
Dr Paul Huggan
BSc (Hons) MBChB FRACP FRCP (Edin)
Clinical Director at the Department of Infectious Disease
Waikato Hospital and District Health Board
Abstract
Sepsis is defined as a life-threatening disorder caused by a dysregulated immune response to infection. It can be a challenging condition to recognise, and patients with sepsis are some of the most complex in any medical or surgical service. Sepsis patients are demanding due to their illness severity and underlying morbidities. Successful resuscitation relies on recognition, early delivery of appropriate anti-microbial therapy and management of shock/organ failure. Parallel efforts are required to identify and treat the infection driving the sepsis response, often leading to multiple investigations, procedures, and long stays in hospital. Ongoing efforts are required to recognise and treat sepsis as the population vulnerabilities that drive infection persist in New Zealand.
Introduction
Infectious diseases are the most common cause of hospitalisation in New Zealand (1). Rates of infection-related hospitalisation have been increasing for several decades (1). Infectious diseases and sepsis are more common amongst older adults, Māori and Pacific people, and those living in conditions of high socio-economic deprivation (2). These are independent risks – for example, non-Māori people living in settings of deprivation are also at increased risk (3). Sepsis presentations mirror infectious disease epidemiology – where there is more infection in a region or population, there is more sepsis (2).
Presentations with sepsis present to every sub-specialty of medicine or surgery. Because every specialty deals with some sepsis, counting cases has to be done using administrative data. In a study of sepsis epidemiology in the Waikato region, we took patients with primary admission codes for major infectious diseases, then identified the sickest patients in this group by looking for secondary codes describing organ failure. We found that 8% of all admissions over a five year period had a primary infectious disease code, of which 10% had organ failure codes (2). That means that about 1% of all admissions met this definition of sepsis. The 28-day mortality in this group was almost 19%, increasing to 37% in patients admitted to our intensive care unit (ICU).
Case vignette one
Mr M, a 61-year-old man, has been admitted to hospital for management of hemiparetic stroke. He has benign prostatic hypertrophy and was catheterised two days ago for new urinary retention. You are asked to see him with a fever of 38.50C. He has rigors and is confused. You find his peripheries are warm, pulse 115 beats per minute, respiratory rate 28 breaths per minute, systolic blood pressure (SBP) 110 (normal SBP in hospital has been 160), SpO2 95% on room air. He has no signs of peripheral line infection. There are no abnormalities on auscultation of the chest. He has a soft abdomen and a small amount of concentrated urine is in the catheter bag.
-------------------------------------------------------------------------------------------------------------------------------------
Box 1. Definitions of sepsis and related terms.
Sepsis 3.0 narrative definition (proposed in the Third International Consensus Definitions for sepsis and septic shock) (4):
“Sepsis is characterised by a life-threatening organ dysfunction due to a dysregulated host response to infection.”
Lay narrative definitions of sepsis:
- “Sepsis is a life-threatening condition that arises when the body’s response to an infection injures its own tissues and organs.” (4)
- “Blood poisoning.”
- Sepsis: Mate whaktāoke (mate = illness; tāoke = toxin; whaktāoke = to make toxic).
- Blood poisoning: Toto pirau (toto = blood; pirau = festering/infected).
“An acute change in total Sequential Organ Failure Assessment Score (SOFA) ≥2 points consequent to infection.”
Septicaemia:
An outdated (but commonly recognised) term indicating a bloodborne inflammatory response due to infection.
Bacteraemia:
The presence of bacteria in the blood.
Hypoperfusion:
Reduced flow of blood through tissue.
Hypotension (in adults):
- SBP <90 mmHg.
- A drop in systolic blood pressure of ≥40 mmHg from the patient’s normal.
- a mean arterial pressure <65 (the mean arterial pressure is the average blood pressure in a patient’s arteries over one cardiac cycle – in the absence of invasive monitoring, calculate by doubling the diastolic blood pressure, adding to the systolic blood pressure and dividing this number by 3).
A severe variant of sepsis that has an in-hospital mortality in excess of 40%.
Narrative definition (4): “a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality.”
Clinical definition (4): “persisting hypotension requiring vasopressors to maintain MAP ≥65 mmHg plus a serum lactate level >2 mmol/L despite adequate volume resuscitation.”
--------------------------------------------------------------------------------------------------------------------------------------
What is sepsis and how is it recognised?
Current definitions of sepsis, septic shock and related terms are given in Box 1.
Biologically, sepsis is best conceived as an acute inflammatory disease of the circulation caused by infection, or “malignant intravascular inflammation” (5). Disturbance of endothelial and circulatory function (further described below) leads to tissue injury, which accumulates and progresses over time. This is why the earliest signs of sepsis can be non-specific, but then evolve quickly. Presentations can vary depending on the patient, the stage of the illness at presentation, the nature of the underlying infection, variable manifestations of organ failure, and, to a certain extent, the micro-organism involved.
If sepsis-related tissue injury goes untreated (i.e. with antimicrobials, management of the infection, and careful supportive care) it gets harder to manage as time passes, while at the same time, the underlying infection becomes increasingly obvious. This is literally a classic medical dilemma, described succinctly by Niccolo Machiavelli in ‘The Prince’, written in 1532:
"As the physicians say it happens in hectic fever, that in the beginning of the malady it is easy to cure but difficult to detect, but in the course of time, not having been either detected or treated in the beginning, it becomes easy to detect but difficult to cure."
Our patient Mr M appears very unwell and sepsis seems likely, but the underlying infection has not yet been identified. At this stage, the formal “Sepsis 3.0” (see Box 1) definition of sepsis cannot be applied, as this requires laboratory testing. Guidelines published in New Zealand by the Best Practice Advocacy Centre (BPAC) recommend the use of available findings to categorise patients as being at a high, moderate, or low risk of sepsis (6). The New Zealand Sepsis Trust (NZST) has collaborated with the UK Sepsis Trust (UKST) to publish a Sepsis Screening and Action Tool (Figure 1), which simplifies the assessment process. It is available at www.sepsis.org.nz and uses the presence of Red Flags to justify rapid investigation and treatment. Red Flags cannot easily be memorised, but note that in non-pregnant adults, any single vital sign that would score a 3 using the national early warning score (EWS) counts as a Red Flag. Paediatric and maternal physiology differ, which is why there are specific tools for these groups (available at www.sepsis.org.nz). Some patients with less severe abnormalities (Amber Flags) are still at moderate risk of progressing to sepsis and need careful assessment.
Mr M has several Red and Amber Flags. He has new onset confusion, his nurse is worried about his condition, he has dropped his SBP greater (40 mmHg below his normal), and he has a respiratory rate of 28 breaths per minute. He needs immediate investigation and treatment using “Sepsis Six” (Figure 2). This is printed on the reverse of the Sepsis Screening and Action Tool, focusing attention on securing a diagnosis and administering effective antibiotics.
Case vignette two
The house officer and nurse both attended sepsis training as part of their hospital orientation. They use the screening and action tool to confirm Red Flag sepsis. The house officer checks the local antimicrobial guideline for sepsis. Because of recent catheterisation in a male, and in the absence of any other clinical signs, she looks for recommendations for treating sepsis of urinary source. Ceftriaxone and gentamicin are recommended. She asks the nurse to prepare these while she inserts a new intravenous line, drawing blood for culture and serum lactate. While antibiotics are being given, the blood pressure is re-checked and confirmed at 105 systolic. The house surgeon decides to give a 500 mL bolus of normal saline over 30 minutes. As the SpO2 is 95% on room air, she decides not to give oxygen, even though the patient’s respiratory rate is elevated. The lab calls a few minutes later to say that the serum lactate is 3.1 mmol/litre (normal range 0.5–2.0).
What are the principles of caring for a patient with sepsis?
Mr M has received prompt treatment with appropriate antibiotics and is being given a fluid bolus. A likely source has been identified and the outlook might be good. However, at this stage it is not clear whether the diagnosis of a urinary tract infection is correct, or indeed whether Mr M will respond appropriately. He needs to be carefully monitored with two things in mind:
- Is the infection correctly identified and treated?
- Is there evidence of persistent hypoperfusion?
The need to keep asking the first question is self-explanatory. The second question needs a little more explanation. The whole immune system is involved in the sepsis response, which is expressed clinically through changes in endothelial and circulatory function. Increased endothelial permeability, neutrophil diapodesis, release of nitric oxide, and migration of inflammatory cells into the interstitium cause tissue oedema and cell damage. When this happens in the skin, the end result is the swelling, redness, heat, and pain (tumor et rubor cum calor et dolore) that can be seen with the naked eye. In sepsis, these changes occur in every major organ, causing tissue damage while also reducing effective circulatory volume (preload) and peripheral resistance (afterload). Damage to the microvasculature combined with low cardiac output leads to global tissue hypoxia. This triggers an increase in production of lactic acid, which in the case of Mr M was elevated above 2 mmol/litre after resuscitation, providing an additional Red Flag.
Available signs of global tissue hypoxia include hypotension, elevated lactate, tachypnoea, and reduced level of consciousness (patient is unresponsive or responds only to voice or pain). If any of these are present at initiation of the Sepsis Six, the patient should be assessed regularly to ensure that things improve. Typically, this means seeing the patient after two to four hours have passed, or earlier if there is reason for concern. NZST have published a Sepsis Hypoperfusion Pathway to aid decision making in this setting. As sepsis is much more common amongst elderly patients, end-of-life decision making may come in to play. However, it is important to recognise that persistent hypoperfusion is a precursor to a poor outcome. Regardless of the underlying situation, involving a senior decision maker or intensive care/medical emergency team is almost always appropriate, even if eligibility for intensive therapies is in doubt.
Case vignette three
Despite a 500 mL fluid bolus, Mr M’s blood pressure continued to fall (to 100 systolic) and his respiratory rate increased to 35 breaths per minute with an SpO2 of 91%. Oxygen was administered via mask and the SpO2 improved to 95%. A further 500 mL fluid bolus was administered but the blood pressure remains unchanged, leading to administration of a 1000 mL fluid bolus over the second hour. By this stage, Mr M has had 20 mL/kg of chrytalloid fluid, but remains drowsy and tachypnoeic. The EWS increases to 10. Despite concerns that the underlying stroke may limit Mr M’s eligibility for intensive care admission, the house surgeon decides to call her registrar, who advises requesting a further lactate and requesting an intensive care review. The intensive care team identify that the blood pressure has fallen further to 90 systolic, there has been no urine produced for four hours and the lactate remains elevated at 4.1 mmol/l. A decision is made to initiate vasopressor support and transfer to the high dependency unit (HDU). The stroke unit consultant attends and confirms that resuscitation efforts are appropriate.
Were the antibiotics appropriate?
Now the situation is looking very serious. Mr M satisfies the definition of septic shock given in Box 1, increasing his risk of in-hospital death to 40%. From the point of view of his antimicrobial therapy, it might be tempting to continue adding “broad spectrum” antibiotics into the mix. However, having sustained a serious inflammatory injury, the body’s response to sepsis can persist in the first few hours, despite effective therapy. This will be particularly true if an abscess, perforation, or other serious anatomical lesion hasn’t been identified and correctly treated (a popular rule of thumb is to “never go to sleep on pus”). With that caveat in mind, if the first clinical assessment is felt to be accurate and antibiotics have been given using an accepted local guideline, it is reasonable to observe response over several hours before considering additional agents. At our hospital, 20% of patients admitted to the ICU or HDU with clinical sepsis have no clearly identified source, but the antibiotics recommended in our guidelines are active in over 95% of cases.
Consider imaging or direct intervention if there might be a drainable collection. Follow the microbiology results carefully. Once susceptibility results are available a decision can be made about how long to continue antibiotic treatment and whether to use an intravenous or oral route.
Clinical vignette four
Mr M spent some time on oxygen and phenylephrine while additional fluid was administered to a total of 30 ml/kg. A chest x-ray showed no pulmonary parenchymal changes. The EWS slowly improved and phenylephrine was weaned overnight. A gram-negative bacillus was identified in blood after six hours of incubation. By the following afternoon this was reported as an Escherichia coli (E.coli), and the same organism was found in a catheter urine specimen, confirming a diagnosis of catheter-associated urinary tract infection. Ceftriaxone was continued for 48 hours, but by the end of the second day, the E.coli was found to be susceptible to amoxicillin. After insertion of a nasogastric tube, ceftriaxone was stopped and amoxicillin given enterally. Mr M improved quickly and a decision was made to stop antibiotics after seven days. He was transferred back to the stroke unit. The same house surgeon met with the family and was surprised to find that none of them had ever heard of sepsis. As Mr M is going to be discharged with a urinary catheter, his family ask how they would spot sepsis if it happened at home.
How do I talk to patients and families about sepsis?
NZST volunteers surveyed almost 300 people attending the 2019 Fieldays event, and found that only 40% had even heard of the term “sepsis”. Other evidence suggests that these problems of awareness and understanding extend to health care workers, who can struggle to explain sepsis to patients and families. This means that patients often leave hospital with an incomplete understanding of their illness and its consequences. We recommend describing sepsis as “blood poisoning (toto pirau) due to infection”, whether or not people have heard of sepsis. This speaks simply to the severity and pathophysiology of the condition, and provides people with a description they can easily remember and share with others. Many people will have heard of the term “septicaemia”, which was in common use in the medical profession until the early 1990s. It is worth explaining that the preferred term for septicaemia is sepsis.
NZST has produced an information leaflet in English and Te Reo Māori for patients, family, and whānau, available on the health navigator website (search sepsis) and at www.sepsis.org.nz.
What are the unique challenges in managing sepsis in New Zealand?
In the Waikato study, the incidence of sepsis amongst Māori was three times higher than amongst non-Māori. Sepsis incidence is one of a range of health indicators that are worse for Māori than non-Māori (2). This inequality (between group difference in health status) has its origins in inequity (defined as maldistribution of resource), which is a manifestation of institutionalised, interpersonal, and internalised racism (7). Inequity and racism contribute to overcrowding, malnutrition, smoking rates, skin and dental disease, poor health literacy, and difficulty accessing and relating to health care providers.
These, and other factors, not only predispose to infection, but also to serious morbidities that are frequently complicated by infection, for example cancer and rheumatic fever. Sepsis occurs as a complication of infection, ergo, any population with higher rates of infection will have higher rates of sepsis. It would follow that the burdens of sepsis amongst Māori will not be lessened until entrenched health and socio-economic inequities are addressed.
What can I do to prevent sepsis or help those who are recovering from it?
Some deaths from sepsis are preventable with early recognition and response. That is why the mission of the New Zealand Sepsis Trust is to ensure that there are “no preventable deaths due to sepsis in New Zealand”. Significant resources are already devoted to the prevention of infection. Examples include vaccination, sanitation, hand and procedural hygiene, and hospital infection control programs. We also support antimicrobial stewardship efforts. Antimicrobial stewardship programs aim to restrict total antimicrobial use while improving antimicrobial prescribing decisions. While it will always remain important to minimise use of antibiotics in low-risk settings, suspected sepsis is the paramount condition in which early guideline-driven antimicrobial therapy is appropriate.
The NZST’s goals are to ensure: i) that all New Zealanders understand sepsis is deadly, but preventable and treatable; ii) that standardised emergency treatment is provided by clinicians with access to high quality sepsis education; and iii) that sepsis survivors and their families receive support and education to aid their recovery. Sepsis survivors are key partners in our work, helping us to understand the longer term impacts of sepsis on day-to-day physical and cognitive function. For a condition that has twice the mortality of myocardial infarction or stroke, remarkably little has been done to elevate sepsis as a major health concern. You can take part in changing this and find out more at www.sepsis.org.nz.
In summary sepsis is a deadly condition amenable to improved outcomes through increased community awareness, standardised emergency treatment, and efforts to prevent and treat infection in the community.
References
- Baker MG, Barnard LT, Kvalsvig A, Verral A, Zhang J, Keall M, et al. Increasing incidence of serious infectious diseases and inequalities in New Zealand: a national epidemiological study. Lancet. 2012; 379: 1112–9.
- Huggan P, Bell A, Waetford J, Obertova Z, Lawrenson R. Evidence of high mortality and increasing burden of sepsis in a regional sample of the New Zealand population. Open Forum Infect Dis. 2017;4(3): ofx106. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5597865/. DOI: 10.1093/ofid/ofx106
- Huggan P, Wells JE, Browne M, Richardson A, Murdoch DR, Chambers ST. Population-based epidemiology of Staphylococcus aureus bloodstream infection in Canterbury, New Zealand. Intern Med J. 2010;40:117-25.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus fefinitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;23;315:801-10.
- Pinsky MR. Multiple systems organ failure: malignant intravascular inflammation. Crit Care Clin. 1989;5:195-8.
- Best Practice Advocacy Centre New Zealand. Sepsis: recognition, diagnosis and early management (guideline). Dunedin: Best Practice Advisory Centre;2018. Available at: https://bpac.org.nz/guidelines/4/
- Robson B, Harris R (eds). Hauora: Māori standards of health IV. A study of the years 2000–2005. Wellington: Te Rōpū Rangahau Hauora a Eru Pōmare; 2007. Available at: www.hauora.maori.nz
About the author
Dr Paul Huggan, BSc (Hons) MBChB FRACP FRCP (Edin). is Clinical Director, at the Department of Infectious Disease, Waikato Hospital and District Health Board.
Paul trained in acute medicine and infectious diseases in the UK, New Zealand and Singapore before taking up a position in the Waikato. He has a career interest in improving care for severe infection across the spectrum of acute care, leading to the foundation of the New Zealand Sepsis Trust, an organisation dedicated to improving sepsis awareness, clinical care and survivor support.
Acknowledgements
The author would like to thank Dr Katie Walland for reviewing the manuscript prior to submission.
Conflicts of interest
Co-founder and Chair, Sepsis Trust NZ.
Correspondence
Dr Paul Huggan: [email protected]